Transdermal drug delivery systems (TDDS) have emerged as a pivotal focus in pharmaceutical R&D, leveraging their unique advantages to revolutionize medical applications. Microneedle technology (MN), a core component of TDDS, offers a critical pathway to overcome the skin’s stratum corneum barrier and enhance drug penetration efficiency. Hydrogel microneedles, manufactured using PDMS (polydimethylsiloxane) molds, have gained significant attention for their excellent biocompatibility and broad application potential. However, their therapeutic efficacy hinges on the precision of their microscopic structures, imposing stringent requirements on manufacturing techniques.
What Are Microneedles?
Microneedles consist of a microscale needle-tip array affixed to a base, typically measuring 150–1500 µm in length (or even 10–2000 µm) and 50–250 µm in needle-body diameter/width. Unlike traditional injections, their minimal length ensures drug release in the epidermis without reaching the dermis, thus avoiding nerve damage and pain. Evolving from solid microneedles, hydrogel microneedles represent a cutting-edge form: they can be fully removed after drug delivery to eliminate in-vivo residue risks, while offering benefits like high drug loading capacity, superior biocompatibility, and simplified fabrication via PDMS micromolding.
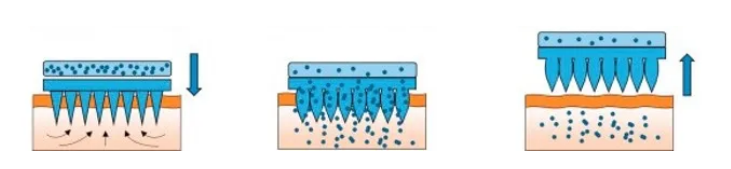
Hydrogel Microneedle Drug Delivery
(Original Image Link: https://doi.org/10.1016/j.mser.2016.03.001)
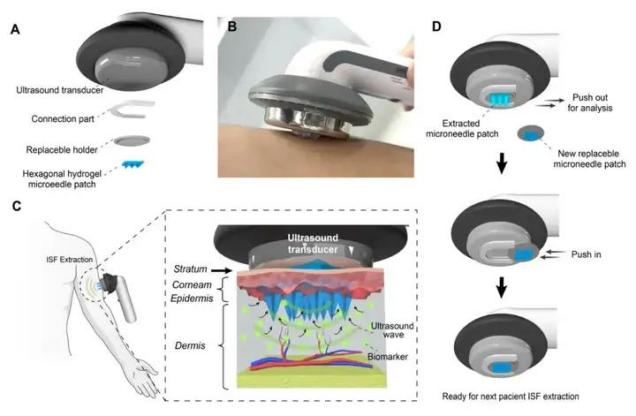
Application Example: Schematic of an ultrasonic-assisted, replaceable hexagonal star hydrogel microneedle patch for skin interstitial fluid collection. (DOI: 10.1016/j.cej.2024.157293)
Crafting Precision on the Needle Tip: A Daunting Challenge
PDMS, a silicone-based polymer, is ideal for hydrogel microneedle molds due to its biocompatibility, low toxicity, flexibility, chemical stability, and mechanical strength in hydrogel formulations. However, these properties also pose challenges: PDMS is prone to shrinkage and deformation during processing, making it difficult to achieve sharp edges and smooth surfaces. 
While 3D printing excels in manufacturing complex 3D structures for personalized applications, it struggles with fine internal features requiring ultra-high surface finish and tip sharpness. Inadequate precision not only hinders skin penetration but also risks material residue blockages. The key challenge: How to fabricate micron- or nanometer-scale needle arrays on soft, elastic PDMS?
Femtosecond Laser Technology: A Breakthrough Solution
Femtosecond laser processing addresses these challenges through its unique physical mechanisms, enabling precision manufacturing of PDMS hydrogel microneedles:
1、"Cold Processing" for Deformation-Free Fabrication
Biomedical microdevice manufacturing demands strict control over thermal effects to prevent material damage or contamination. Femtosecond lasers operate via non-contact "cold processing": their ultra-short interaction time with materials ensures energy is absorbed and vaporized before heat transfers to the lattice. This minimizes the heat-affected zone (HAZ), eliminating issues like melting, thermal deformation, and carbonization common in polymer processing. The result: sharply defined edges and precise structures, perfectly suited for soft, heat-sensitive materials like PDMS.
2、Sub-Micron 3D Fabrication Capability
Needle-tip sharpness and mechanical strength are critical for stratum corneum penetration, while uniform geometric parameters (height, taper, aspect ratio) across the array ensure consistent drug distribution and predictable 疗效 (therapeutic outcomes). Femtosecond lasers, guided by computer-controlled beam scanning, directly etch designed microneedle arrays onto PDMS substrates. This enables fabrication of complex 3D microstructures—including adjustable-taper needle tips—with ±1 µm precision and uniform array height.
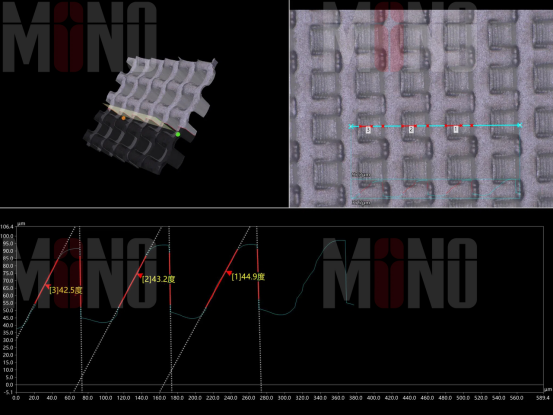
Example: Femtosecond laser etching of 45° ramp bump microstructures with ±2 µm precision.
Application Pathways for Femtosecond Lasers in Hydrogel Microneedle Fabrication
1、Direct Etching for R&D and Prototyping
Femtosecond lasers offer unmatched flexibility through direct-writing technology: software-defined scanning paths enable precise fabrication of microneedle arrays on various polymers, alongside integrated ultra-precision operations like drilling and cutting. This streamlined process is ideal for rapid prototyping in R&D and personalized microneedle design.
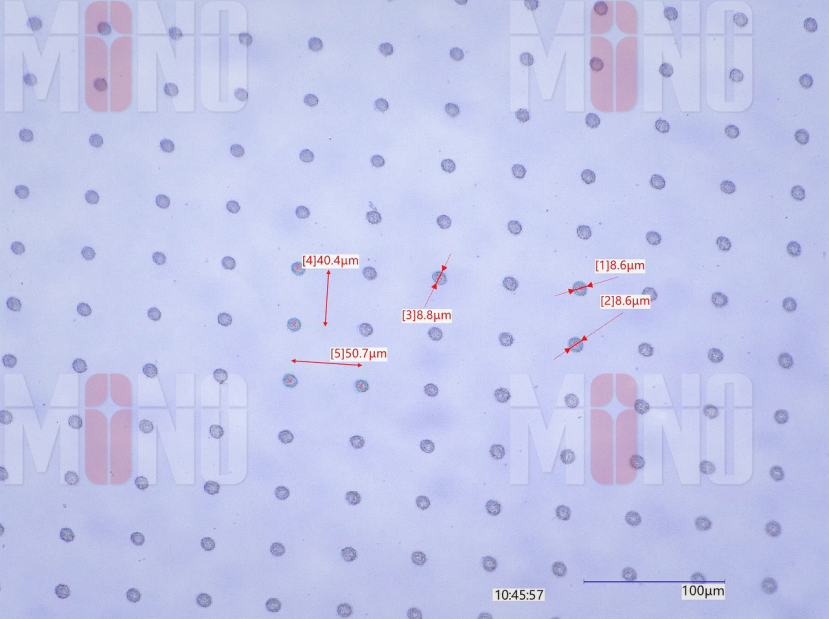
Example: Femtosecond laser machining of an 8-µm aperture in PDMS film with ±2 µm precision.
2、High-Precision Mold Fabrication for Mass Production
For large-scale manufacturing, femtosecond lasers create PDMS molds with sub-micron accuracy and ultra-smooth surfaces, eliminating issues like polymer residue and structural inconsistency. These molds ensure stable drug loading and release, while subsequent replication molding enables cost-effective, high-volume production of hydrogel microneedles.
Future Outlook
Microneedle-based transdermal delivery is widely used in drug and vaccine administration, as well as aesthetic medicine for skin rejuvenation and treating conditions like acne, melasma, and hair loss. As femtosecond laser technology advances, its role in manufacturing high-end biomedical microdevices—especially precision microneedle systems—will deepen, driving TDDS toward greater precision, efficiency, and personalization.
